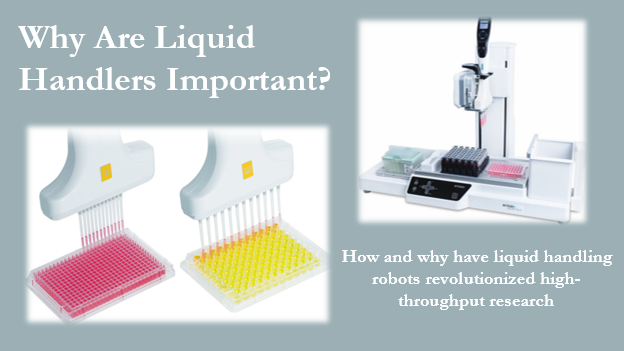
Legend: Some common uses of liquid handlers are shown above. The right image is an example single pipette liquid handler that can be programmed to transfer a single defined volume of liquid at a time from different sources to the multi-well plate on the right. The image on the left shows multichannel pipettes that can be used by other liquid handlers to increase throughput on 96- or 384-well plates.
Background and Introduction
Powerful tools are one of the main aspects of human life that differentiates us and our societies from the rest of the animal kingdom. Liquid handlers are an example of one such type of tool that allows us to better understand and explore our world. Liquid handlers (or liquid handling robots) are modular tools used in life science laboratories which are used to transfer specifically programmed volumes of fluids in a systematic order. Liquid handlers are typically used in high-throughput research contexts to lower overall research time, human error, and variation. However, there are many different factors to consider when deciding to use liquid handlers including cost, time, and experimental conditions. Liquid handlers have proven themselves invaluable across many different types of high-throughput studies and will continue to find increased use in the future.
Before liquid handlers were invented, small liquid volumes were transferred using micropipettes which were invented in the late 1950’s by Heinrich Schnitger (1). These micropipettes revolutionized many fields related to and including biotechnology which then began to develop rapidly. Before this, moving small volumes of liquids involved imprecise glass capillary tubes being produced in a lab and then mouth pipetted, potentially causing the inhalation of dangerous fumes (1). Micropipettes were thus both a safety and technical improvement over previous imprecise methods of moving nano- or micro-liters. Following the creation of manual micropipettes, the next large leap in pipette technology was robotic liquid handlers which combines the technical accuracy of micropipettes with the automated low-variance repetition of robots.
Robotic liquid handlers were first invented and used by the 1980’s with significant adoption occurring in the 1990’s, especially by pharmaceutical companies looking to vastly increase the compounds that can be screened quickly (2). The preciseness, speed, and versatility of liquid handling robots led to an increased usage in other fields, especially biotechnology where liquid handling robots are used for various high-throughput applications. High-throughput screening in this context is used to speed up identification of candidate genes, compounds, proteins, etc. that show activity within a specific assay.
Uses, Requirements, and Drawbacks of Liquid Handlers
Liquid handlers have a wide variety of uses and may be applied to solve large-scale experimental questions quickly, reliably, and economically. A recent study screened natural compounds for their ability to inhibit SARS-CoV-2 (COVID-19) which involved liquid handlers managing a fluorescent protease reporter, a large (1058) compound library, and Vero cell line to determine cytotoxicity (4). From this study, 30 hits were found showing antiviral activity which might allow later deeper research into these compounds (4). Another example is from an earlier study which randomly mutated a specific glycosyltransferase and used liquid handlers to assay the mutant enzymes substrate promiscuity via a fluorescent marker (5). This allowed for the identification of specific amino acid substitutions based on which mutant enzymes had increased activity (5). From these 2 examples it is clear that the high-throughput benefit of liquid handlers on many studies allows for a quick reproducible assay across a variety of conditions.
Quality control (QC) of liquid handlers is necessary to guarantee correct application, data reproducibility, and interpretation of high-throughput assay results. QC involves confirmation of proper dispensing volume by liquid handlers and microplate washing to eliminate background noise (3). Jones et al., 2003 explains how liquid handling dispensing volume and regularity can be confirmed by a few methods (3). One method is addition of a known volume of water and weighing the plate before and after dispensing. However, this method may not be able to pinpoint where error is coming from, especially if a multichannel pipette is being used. Another QC method is colorimetric confirmation by dispensing a known amount of tartrazine (or other dye) in increasing volumes to compare to a standard curve via absorbance from a plate reader which could better identify where problems are appearing. This method is not absolute as it must be compared to a standard curve that was hand pipetted onto an empty plate, thus an assumption must be made that there is little to no human error and that the manual pipette is properly calibrated when preparing the standard curve.
While there are many uses for liquid handlers, there are also many situations and assays where they may not be required or valuable enough to warrant their use. Depending on the assay or lab, the overall cost of purchasing or renting a liquid handling machine may not make sense. Full Liquid handler workstations can cost anywhere from $50,000-$250,000, not including the cost of required reagents/consumables, making them inaccessible to most low- to mid-range labs (6). Due to the high cost of purchasing full workstations, many labs instead choose to use screening centers or choose cheaper ($5,000-$30,000) open-source liquid handling robots (6). Another concern for labs is the time it may take to optimize a high-throughput protocol. In addition, the time and cost of maintenance and machine error need to be considered by each lab when considering the overall value it will provide given potentially years of use. While the cost and time drawbacks are significant enough to make any lab question the utility a liquid handler will provide them, the many benefits usually outweigh these downsides.
Significance of Liquid Handlers
Liquid handlers have come a very long way in a short amount of time. Robotic liquid handlers as we know them only really came into existence in the 1980’s and were accepted by major companies in the 1990’s (2). Full workstations are currently available at high costs, but recently cheaper open-source and DIY versions have allowed even smaller labs access to high-throughput research (6). This technology has provided for more precise and reliable pipetting as an alternative to human error, especially when continued repeated actions are required. Liquid handlers have also been allowed to further develop by the miniaturization of lab techniques and tools, thus reducing the amount of required materials and space per experiment for lower overall cost (7).
Liquid handlers have enabled many large-scale experiments that otherwise would have been far too expensive, slow, and/or potentially unsafe for manual pipetting. For large scale projects where hundreds or thousands of compounds/treatments are performed on a few targets/cells, it makes much more sense to use a high-throughput liquid handler. Manual pipetting is still preferable to liquid handlers when an experiment requires a wide variation of compounds, cells, treatments, etc. as robots are currently better developed for extended repeated actions. As such, liquid handlers can make precisely replicated movements for very long periods of time with little to no drop in efficiency or error. In addition, liquid handlers can deal with toxic compounds or dangerous viruses that could potentially endanger lives if it were performed by a person. Liquid handlers fit this niche of large-scale high-throughput scientific experiments which are not ideal for a humans to accomplish. Thus, liquid handlers represent a huge step forward in the progress of scientific research and enable many unique and important topics that might otherwise be inaccessible or infeasible.
References
- Klingenberg, M. When a common problem meets an ingenious mind. EMBO reports 6, 797–800 (2005).
- Olsen, K. A Short History of Laboratory Robotics. (2005) Accessed: https://msuweb.montclair.edu/~olsenk/robot.htm
- Jones, M. The Importance of the Quality Control of Laboratory Automation. Journal of the Association for Laboratory Automation 8, 55–57 (2003).
- Zhang, Z.-R. et al. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduction and Targeted Therapy 5, (2020).
- Williams, G. J., Zhang, C. & Thorson, J. S. Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution. Nature Chemical Biology 3, 657–662 (2007).
- Tegally, H., San, J. E., Giandhari, J. & de Oliveira, T. Unlocking the efficiency of genomics laboratories with robotic liquid-handling. BMC Genomics 21, (2020).
- Edwards, B., Lesnick, J., Wang, J., Tang, N. & Peters, C. Miniaturization of High-Throughput Epigenetic Methyltransferase Assays with Acoustic Liquid Handling. Journal of Laboratory Automation 21, 208–216 (2016).