IC50’s: An Approach to High-Throughput Drug Discovery
Author: Shouvik Saleh
Learning Objectives
- Describe Drug-Drug Interaction studies and their role in clinical research.
- Understand IC50’s, how they are obtained, and its use in high-throughput settings.
- List some limitations of IC50’s.
Graphical Abstract
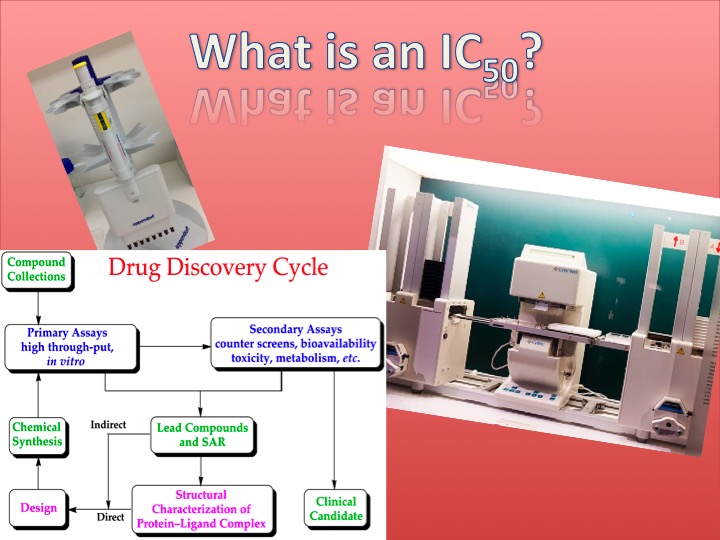
Images retrieved from:
Boghog, https://commons.wikimedia.org/wiki/File:Drug_discovery_cycle.svg
Tiia Monto, https://commons.wikimedia.org/wiki/File:High-throughput_screening.jpg
Andrux, https://commons.wikimedia.org/wiki/File:Multipipet.JPG
Introduction/Background
The rates of biological processes can be affected by the presence of inhibitors or enzymes, but you may wonder – What happens when a drug is added to the equation? How does the quantity and duration of effect of the drug produce changes in these biological processes? This is where IC50’s come in.
DDI Studies
The term IC50 refers to the half maximal inhibitory concentration and is a highly relevant concept for drug discovery and analysis. A study with this might be trying to look at how concentrations of a drug change over time in a body and what interactions occur during that period; in clinical settings, these are called Drug-Drug Interaction (DDI) studies1. There are many different interactions that can occur between drugs and compounds or structures in the body; for example, two drugs may work in concert to produce an additive effect relating to a disease, two drugs may work antagonistically to change the intended effects for a disease, or a new drug and a well-characterized drug with established effects toward a disease or biological process may be used to learn about what kind of interactions the new drug may have in certain conditions.1 A study might look at what maximum and minimum concentrations of a drug are observed as time passes following exposure to a tissue or administration to the body. In these studies, well characterized enzymes, such as cytochrome P450 enzymes, are often used as the target of inhibition, since these liver enzymes are often involved in drug metabolism pathways after administration.
IC50’s in High-Throughput
IC50’s are used in lab settings to estimate the quantities of these drugs that result in a 50% inhibition of a biological process/mechanism or drug interaction specifically in cultured cells.1 These are especially useful for studies looking at novel situations of adding one drug to the body to blunt the effect of another drug, for example; either drug may be the drug of interest for the study here. On the other hand, EC50’s, or the half maximal effective concentration, are used to look at excitatory drug interactions rather than inhibitory ones.2 In essence, both of these measures are of drug potency.
To study these drug interactions in high-throughput, primary screens of chemical libraries consisting of 100,000 to up to 2 million compounds may be tested with cells. To model the interaction of the compounds in diseased humans, proteins observed to be a possible or known cause for the disease may be engineered into cells. These cells are then exposed to compounds from chemical libraries using liquid handlers and their activity is observed over time. The activity is measured before compound addition (at time=0) to get a reading for minimal inhibition from a compound and in time increments until all activity of the cell ceases (at time = infinity) to get a reading for maximal inhibition. A dose-response curve is then made from wells containing cells that show inhibitory effects above a certain threshold, as some compounds may fail at producing an inhibitory effect. The IC50 value is then estimated from the curve using a logistic regression equation, called the 4-parameter logistic Hill equation, used in dose-response relationships.2
Limitations
There can be limitations to this process however. There can be variability in concentrations between samples due to liquid handling and characteristics of the reagents being used; inconsistencies in reported data being used for parameters used for calculations due to interactions between reagents and the assay being used; and influences on data due to experimental design and quality.3 Since a basic assumption of this high-throughput design is that the percent inhibition and IC50 values correlate reasonably, there can be room left for error when completing calculations with the Hill equation.3 Additionally, inaccuracies have been identified in public collections of compound data due to including below-threshold data.4 It is important that false negatives and false positives are identified to accurately identify compounds that may play potential roles in drug-disease interactions.
Accuracy could possibly be improved by observing compounds at different concentrations but this would also increase the high-throughput demands of the experiment. All in all, IC50’s are an important concept allowing for the responses of pre-made chemical libraries to be observed as they are being exposed to biological compounds or cells. This is important not only for finding potential treatments for diseases, but also for determining the possible side effects of these treatments. High throughput technique enables information about these compounds to be obtained in a time- and resource-efficient manner.
Audio Recording
References
- Brody, T. (2018). “Drug–Drug Interactions: Part One (Small Molecule Drugs).” FDA’s Drug Review Process and the Package Label: Strategies for Writing Successful FDA Submissions, Academic Press, 7(1), 255-335. Accessed: https://doi.org/10.1016/B978-0-12-814647-7.00007-5.
- Sebaugh, J. L. (2011) “Guidelines for Accurate EC50/IC50 Estimation.” Pharmaceutical Statistics, vol. 10, no. 2, pp. 128–134. doi:10.1002/pst.426.
- Limitations Gubler, H., Schopfer, U., & Jacoby, E. (2013). “Theoretical and Experimental Relationships between Percent Inhibition and IC50 Data Observed in High-Throughput Screening.” Journal of Biomolecular Screening, 18(1), 1–13. Accessed: https://doi.org/10.1177/1087057112455219.
- Kalliokoski, T., Kramer, C., Vulpetti, A., & Gedeck, P. (2013). “Comparability of mixed IC₅₀ data – a statistical analysis.” PloS one, 8(4), e61007. https://doi.org/10.1371/journal.pone.0061007
Questions
- What are IC50’s used for? These are used to look at the inhibition of a drug or biological process as a result of interruption or slowing caused by drug interactions from another drug.
- What is needed to calculate an IC50? Minimal and maximal activity levels of cells following exposure to a constant concentration of compound
- What are some applications for IC50’s in high-throughput studies? They can be used alongside chemical libraries with cells for high-throughput drug discovery and identification.
- What is a possible interaction between drugs that might be seen in a DDI study for which IC50’s would be inappropriate? They cannot be used to look at non-inhibitory interactions, since the measurements are looking at decreases in activity of cells.
- What are some limitations to IC50’s in high-throughput applications? They require optimization based on many characteristics related to the assay and are susceptible to errors due to possible missed recordings of variations in data.